Abstract
Heparin-induced thrombocytopenia (HIT) is a life-threatening immune-mediated thrombotic disorder caused by immune complexes (ICs) containing IgG antibodies to platelet factor 4 (PF4)/heparin complexes. In previous studies, we established that complement opsonization of HIT ICs is essential for cellular binding of ICs and subsequent engagement of Fcγ receptors (FcγRs). We additionally showed that complement inhibition alone prevented FcγR-mediated effector functions, such as neutrophil degranulation, monocyte tissue factor expression, and procoagulant activity (Khandelwal, Blood 2021). As KKO and HIT ICs can also bind FcγRs in media without complement, we undertook these studies to examine the direct effects of complement on IC-FcγR interactions.
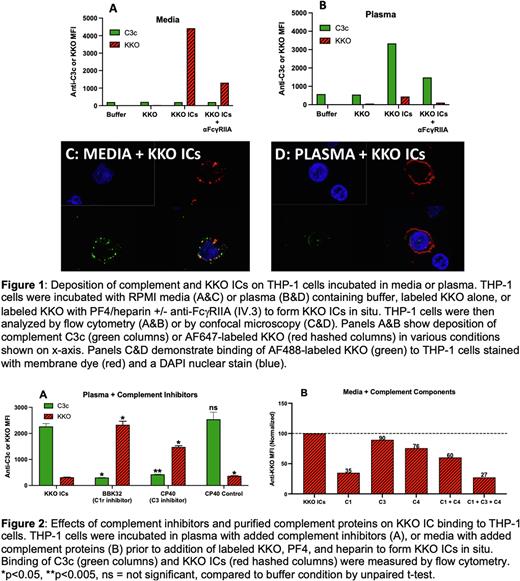
For these studies, we used THP-1 cells or U937 cells, monocytic cell lines expressing FcγRI and FcγRII, but not complement receptors (CRs). Cells were incubated with AF-647 labeled KKO (50 mg/ml), a HIT-like monoclonal antibody, either alone or with PF4 (25 mg/ml) and heparin (1U/ml) to form in situ KKO ICs. Labeled KKO or KKO ICs were added to RPMI-containing media (lacking complement) or undiluted citrated plasma (source of complement) in the presence or absence of anti-FcγRIIA. Binding of labeled IgG or anti-C3c was examined by flow cytometry. Consistent with prior observations, THP-1 cells incubated in media show robust binding of KKO ICs in a FcγRII dependent manner (70% reduction in binding with anti-FcγRII; Figure 1A). Surprisingly, in undiluted plasma, there was minimal KKO IC binding to THP-1 cells, despite adequate complement generation (Figure 1B). Similar effects of plasma vs. media were seen in confocal experiments using KKO ICs with THP-1 cells (Figure 1C & 1D), HIT ICs incubated with THP-1 cells (containing HIT patient IgG, PF4/heparin; data not shown), or KKO ICs incubated with U937 cells (data not shown). In other studies, we note that binding of KKO ICs was dependent on the source of complement, with undiluted human plasma showing maximal inhibitory effects (100%), while undiluted normal human serum (NHS) and fetal bovine serum (FBS) reduced KKO IC binding by 70% and 50%, respectively (data not shown). To demonstrate that the inhibitory effects of undiluted plasma on KKO IC binding was complement-mediated, THP-1 cells were incubated in plasma containing the complement inhibitors BBK32 (C1r inhibitor) or CP40 (C3 inhibitor) prior to addition of labeled KKO ICs. In other experiments, THP-1 cells were incubated with labeled KKO ICs in media containing human complement proteins (C1+C3+C4). As shown, complement inhibition in plasma restored KKO IC binding to cellular FcγR (Figure 2A). Conversely, addition of purified complement components to media resulted in reduced KKO IC binding (Figure 2B). Similar effects of complement proteins and inhibitors were noted in ELISA-based assays using immobilized FcγRIIA incubated with KKO ICs in plasma or media (data not shown).
These studies demonstrate striking inhibitory effects of complement on IC-FcγR interactions. Specifically, complement shields Fc regions on ICs from recognition and binding to cellular FcγRs and this masking effect is reversed by complement inhibition. We additionally show that these masking effects of complement are less apparent in media containing FBS, the most commonly used source of complement for cell-based studies. Taken together with our recent observations showing requirements for complement receptors in binding complement-opsonized KKO ICs, these studies suggest that the masking effects of complement on ICs prevent FcγR activation on cells lacking CRs.
Disclosures
Khandelwal:Alexion Pharmaceuticals: Research Funding; Annexon Biosciences: Research Funding; BTG International Inc.: Research Funding. Rauova:Astra Zeneca: Research Funding; Rigel Pharmaceuticals Inc: Research Funding. Arepally:Veralox: Consultancy; Biokit: Patents & Royalties; AstraZeneca: Consultancy; Alexion Pharmaceuticals: Research Funding; Annexon Biosciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal